Co-Chairs: Brad Elder, MD, and Gavin Dunn, MD
Members: Dan Cahill, MD, Melanie Hayden Gephart, MD, Mark Linskey, MD, Timothy Smith, MD, Andrew Sloan, MD, and Michael Vogelbaum, MD
From the “Clinical Trials and Registries” Committee, we are excited to provide updates on important tumor registries and highlight clinical trial activity. In addition to this update, we are planning to send a survey to neurosurgeons who specialize in neurosurgical oncology. This survey aims to gauge involvement and interest in national clinical trials and tumor outcomes registries. The survey should be brief, and we are targeting the maximal engagement of our peers. Please look for this survey in your email in the coming months.
Clinical Trials in Neuro-Oncology
Important dates:
NRG Winter Meeting: Orlando, Fla., February 15-17, 2024
Alliance Spring Meeting: Chicago, Ill. (hybrid meeting), May 15-18, 2024
NRG: Denver, Colo., July 25-27, 2024
Alliance Fall Meeting: Chicago, Ill. (hybrid meeting), October 30 – November 2, 2024
In the Fall 2023 Report, we highlighted the NRG study NRG-BN012, “A randomized phase III trial of pre-operative compared to post-operative stereotactic radiosurgery in patients with resectable brain metastases”.
In this report, we highlight the Alliance trial “A071401, Phase II Trial of SMO/ AKT/ NF2/CDK Inhibitors in Progressive Meningiomas with SMO/ AKT/ NF2/CDK Pathway Mutations.” Inclusion criteria are patients with progressive meningiomas harboring SMO, AKT, NF2 or CDK pathway mutations. The primary objective is to determine the activity of inhibitors matched to each of the four mutation pathways. This includes vismodegib (SMO/PTCH1 mutation), FAK inhibitor GSK2256098 (NF2 mutation), capivasertib (AKT1, PIK3CA or PTEN mutation) and abemaciclib (CDK4, CDK6, CDKN2A, CCND1, CCND2, CCND3, CCNE1 mutation). Target enrollment is 124 patients. PI is Priscilla Brastianos, MD.
Further information regarding this clinical trial can be obtained from the NeuroPoint Alliance website at or clinicaltrials.gov.
NeuroPoint Alliance
Background:
The NeuroPoint Alliance (NPA) was established in 2008 to improve quality of care for neurosurgical patients through acquisition and analysis of clinical outcomes data. The goals of the NPA include:
- Establish risk-adjusted national benchmarks for the safety and effectiveness of neurosurgical and spine procedures.
- Allow practice groups and hospitals to analyze their individual morbidity and clinical outcomes in real time.
- Generate quality and efficacy data to support claims made to public and private payers.
- Demonstrate the comparative effectiveness of neurosurgical procedures.
- Facilitate essential multi-center trials and other cooperative clinical studies.
Participating in the Tumor QOD Registry
Participating institutes can include academic medical centers, hospitals, ambulatory surgery centers, health care systems, private practice groups and individual surgeons. The startup process includes registration, training and onboarding and is followed by continuous site support and monitoring.
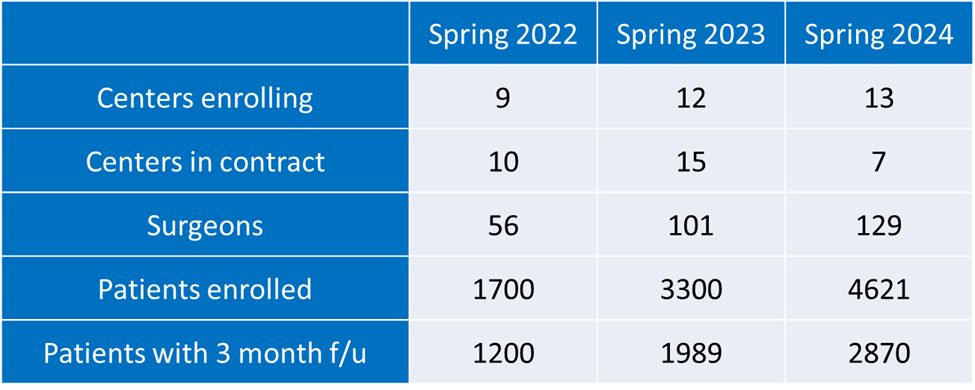
The current price point is a three-year contract at $5,000/year registration fees, plus funding of a 0.5 FTE for data entry. Patient accrual and startup of interested neurosurgical groups has increased steadily since the Tumor QOD opened.
Those interested in more information regarding participating in the NPA Tumor QOD should contact Brad Elder at brad.elder@osumc.edu